Carbon for Hydrogen Production and Delivery
Advanced Renewable
Wed , 06 Mar 2024 21:25 WIB

In a previous upload (https://lnkd.in/g6PJJWkF) I shared data showing that charcoal can be a hydrogen carrier with the highest delivery potential compared to other carriers, especially compared to pure hydrogen.
Compared to other existing solid carriers, namely magnesium, charcoal has a 5x higher delivery capacity. Compared to ammonia and methanol, it is 2x higher, and compared to ethanol and DME, charcoal has a delivery capacity 1.5 times higher. Charcoal logistics costs are also definitely much cheaper than all of other types of hydrogen carriers mentioned above.
By using charcoal as a hydrogen carrier, the hydrogen production pattern is also changed. From being produced centrally and then shipped very expensively to the user's location, to being produced in-situ and in-time, on the spot and when the user wants to use it.
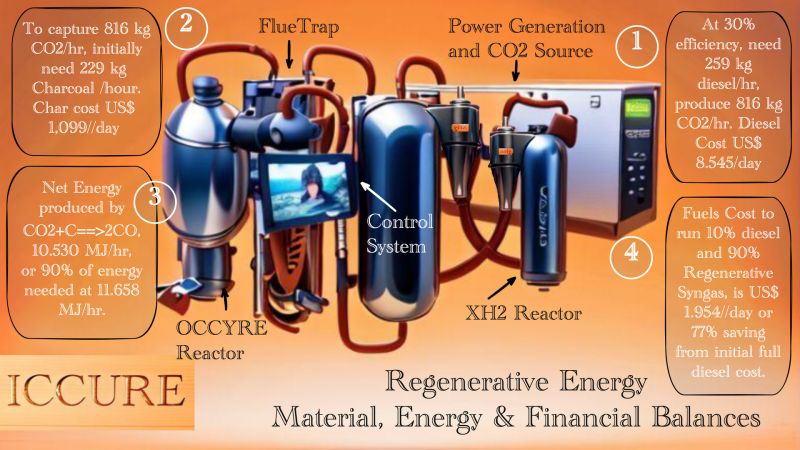
Because there will be a need for small hydrogen production units, but as many as users, this hydrogen production unit must be made in a compact and scalable form according to user needs. Our AI-based design is in the picture below.
Similar to the previous reactor which we call XH2, the difference is - this reactor for hydrogen production is equipped with a membrane reactor. The essence of the process is a combination of Water Gas (WG) and Water Gas Shift (WGS) reactions, plus purification.
We derive from these two chemical reactions into a simpler formula which we call Carbon To Gas (CTG) reaction, because after these reactions the two products are all gas, C + 2H2O==> 2H2 + CO2. Hydrogen (H2) is the product we want, while CO2 is the emissions we capture back using FlueTrap and OCCYRE technology to become CO gas. The CO gas can be used to increase Hydrogen production again using the same reactor under the WGS reaction.
Overall, the CTG reaction mentioned above is very energy efficient because the reaction is only slightly endothermic, 90 kJ/mol C. To produce 1 kg of H2, for example, only requires around 6 kWh of energy with the resulting H2 energy content being 34 kWh/kg. Compare this with H2 via the electrolysis process, which requires 53 kWh of electrical energy to produce the same energy of 34 kWh/kg.
So not only will the costs of storing and shipping H2 be greatly reduced by using H2 on demand which is produced in-situ and in-time with charcoal feedstocks, but also in terms of energy requirements for the process which is very low compared to other hydrogen production processes.
These reactors can be produced on an order basis, but we are looking for global partners for mass production, so that it will be much cheaper per unit. If these reactors can be produced cheaply, then Clean and Affordable Energy (SDGs no. 7), will really be achieved long before the SDGs year 2030!
Pos Lainnya
Jika Carbon Bisa Bicara
Mar 06, 2024
Tanpa Sumbu, Tanpa Tabung
Mar 06, 2024
Green Hydrogen Cost Driver
Mar 06, 2024
Solution for FEW Trilemma
Mar 06, 2024
Energi Regeneratif: Material, Energi dan Finansial
Mar 06, 2024
Kategori
Renewable Energy





Silakan mendaftar terlebih dahulu!
Untuk memposting komentar baru. Anda harus login terlebih dahulu. Masuk
Komentar
Tidak ada komentar