Race Towards Green Hydrogen Delivery
Advanced Renewable
Wed , 06 Mar 2024 21:15 WIB
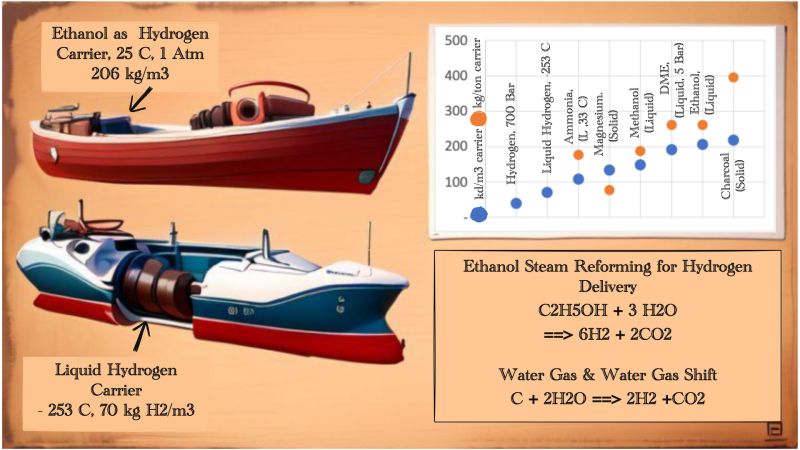
One of the clean energies that the world has been waiting for is green hydrogen. Many technologies for truly green hydrogen production have been proven, such as the use of renewable energy from hydropower, wind, etc. for water electrolysis.
The problem is that even if hydrogen is produced greenly, delivering it to end users is neither easy nor cheap. Hydrogen transported in tanks with a pressure of 700 bar, can only transport around 40 kg of pure hydrogen per m3. Even at an extra low temperature of minus 253 degrees Celsius, liquid hydrogen can only be transported around 70 kg/m3.
So various cheaper hydrogen carrier technologies emerged, the graph below is the technology options currently available. Some use ammonia, with a capacity of 107 kg/m3. Using solid magnesium in the form of MgH2, with a capacity of 134 kg/m3.
The next technology is to use oxygenates as carriers that can deliver more hydrogen than it carries itself. The choices are methanol, ethanol and DME. All three will get additional hydrogen from the steam used for reforming the oxygenates. Like the example of ethanol reforming formula below. Methanol, ethanol and DME are capable of delivering H2 up to 149 kg/m3, 206 kg/m3 and 192 kg/m3 respectively.
From here we can see that oxygenates such as methanol, ethanol and DME have the potential to become H2 carriers which are much more effective than other carriers such as ammonia and magnesium, especially superior as compared with pure hydrogen at a pressure of 700 Bar or a temperature of minus 253 degrees Celsius.
On top of that, our research show that there is other carrier that is much more effective and much cheaper, even compared to carriers of the oxygenates. We use charcoal for this new carrier with special treatment. Charcoal, which generally already carries hydrogen in the range of 3 - 8%, can be increased to deliver H2 up to 40% of the weight of the charcoal through two processes, namely Water Gas (WG) and Water Gas Shift (WGS) reaction.
The chemical reaction equation that we derived from WG and WGS becomes what we call the Carbon To Gas (CTG) reaction, with the formula C+2H2O==>2H2+CO2. We can see here that carbon can directly deliver hydrogen with the help of steam, from this reaction we can also see how charcoal is able to deliver much more hydrogen than other hydrogen carriers.
With this charcoal as H2 carrier, actually H2 is produced in-time and in-situ, when and where it is needed. Apart from hydrogen will have a very low cost in production and delivery, the carbon footprint can be reduced to its lowest point. What is needed for this CTG system is a special reactor for the WG and WGS processes mentioned above, plus a FlueTrap to capture CO2 emissions from the processes. I will share the reactor design in the next upload.
Pos Lainnya
Halophyte For Advanced Biofuels
Mar 06, 2024
Si Hitam Yang Bisa Menghadirkan Ekonomi Hijau
Mar 06, 2024
3 Steps For Net-Zero Emission
Mar 06, 2024
Universal Green Fuels and Feedstocks
Mar 06, 2024
Appearance of the Regenerative Energy Reactor
Mar 06, 2024
Kategori
Renewable Energy





Silakan mendaftar terlebih dahulu!
Untuk memposting komentar baru. Anda harus login terlebih dahulu. Masuk
Komentar
Tidak ada komentar